by Raphael Wurm
Our paper of the month for May is Jia Jianping, Ning Yuye, Chen Meilin, et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. New England Journal of Medicine. 2024;390:712–722. DOI: 10.1056/NEJMoa2310168.
We know from seminal work from Clifford Jack and colleagues that in genetic forms of Alzheimer’s disease (AD), biomarkers emerge many years before the hypothetical onset of the disease. In this older trial, the researchers exploited the knowledge that patients with genetic AD will have almost the same year of onset as their affected parent, allowing them to calculate the years to onset in a cross-sectional study.
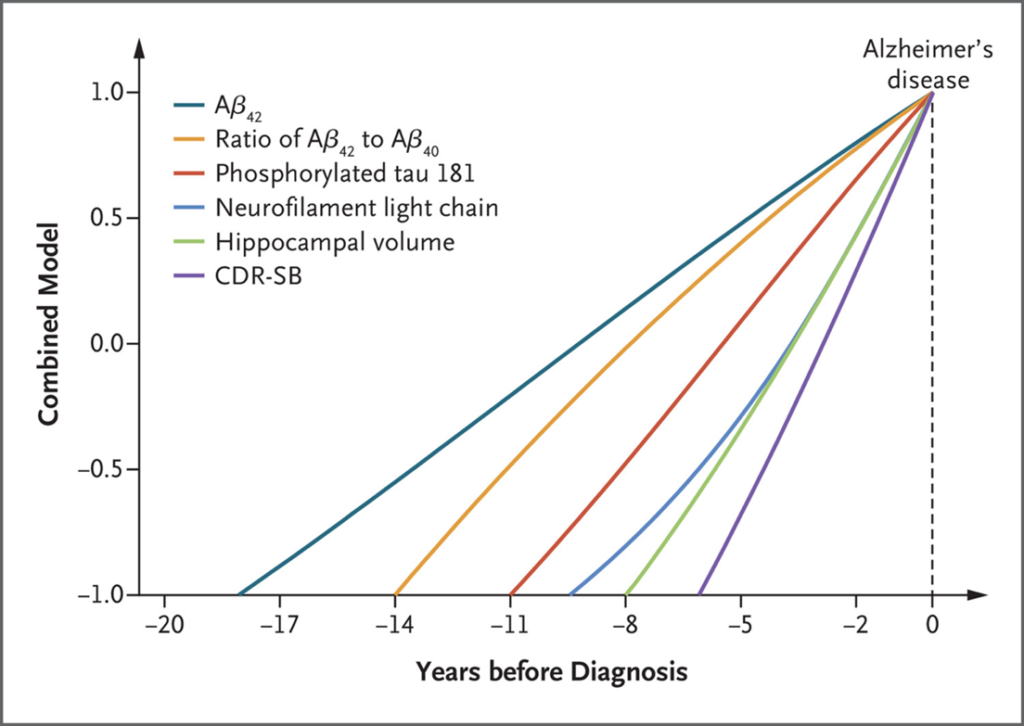
In a landmark study now published in the New England Journal of Medicine, Chinese researchers meticulously traced the trajectory of AD biomarkers over time, for the first time employing a longitudinal design. The study was conducted under the umbrella of the China Cognition and Aging study, which is a large, prospective cohort study that spans 20 years. It enrolled 52,000 participants in the first half of 2000, with a focus on individuals of Han Chinese ancestry. Study participants underwent a comprehensive test battery, including donations of cerebrospinal fluid every two or three years, which created an invaluable biobank for this analysis. Despite the obvious challenges for such a long and intensive study, the researchers were able to identify a group of almost 700 individuals who developed AD during the observational period and matched them to an equal number of unaffected controls. They then traced the emergence of fluid, imaging, and cognitive biomarkers over the study period and recreated the now famous Jack graph.
What they found was both unsurprising and still groundbreaking
We see that the Amyloid beta subunit Aß42 emerges as a signal as early as 18 years before onset, followed by the ratio of Aß42/Aß40 at roughly 14 years. The next marker to change is the phosphorylated tau at position 181 (pTau181). Ten years prior to diagnosis, neurofilament light chain starts to rise which is shortly followed by changes in hippocampal volume (assessed by automatic volumetry). Clinical disturbances follow very late, and the change in the clinical dementia rating sum of boxes score is only evident some five years before diagnosis.
These findings are important for at least three reasons:
- They confirm with astonishing accuracy what we know from genetic AD cohorts, suggesting that pathophysiologically, the differences between these two diseases might not be as big as we thought.
- They suggest a cascade of changes in which disturbances in Amyloid are the first to occur, followed by Tau, which then leads to neurodegeneration. Only after a decade of these processes, degeneration has progressed enough for even small clinical signs to be evident.
- The window of opportunity for intervention before neurodegeneration occurs is probably at least five years long.
The study has some limitations. The most obvious being the focus on a genetically homogeneous population. However, smaller trials and a recent work that uses a mathematical ‘age to onset’ model, largely confirm these time courses. Another limitation is the availability of samples – both the recruitment of participants and the readiness to provide CSF samples at all follow-ups – leading to a convenient sample that might be selecting for more motivated people. Excluding patients with a family history of AD reduced the numbers of carriers of the APOE4 allele. Together, these limitations diminish the generalisability of the data, but this is eclipsed by the amount of data and the depth of the investigation.
In conclusion, this work has impressively confirmed our knowledge on the trajectories of pathological changes across the AD continuum. It will be an important puzzle piece in our road towards defining the disease biologically and opens the door for preventive efforts and early intervention.