Cross-sectional case-control studies (Blue)
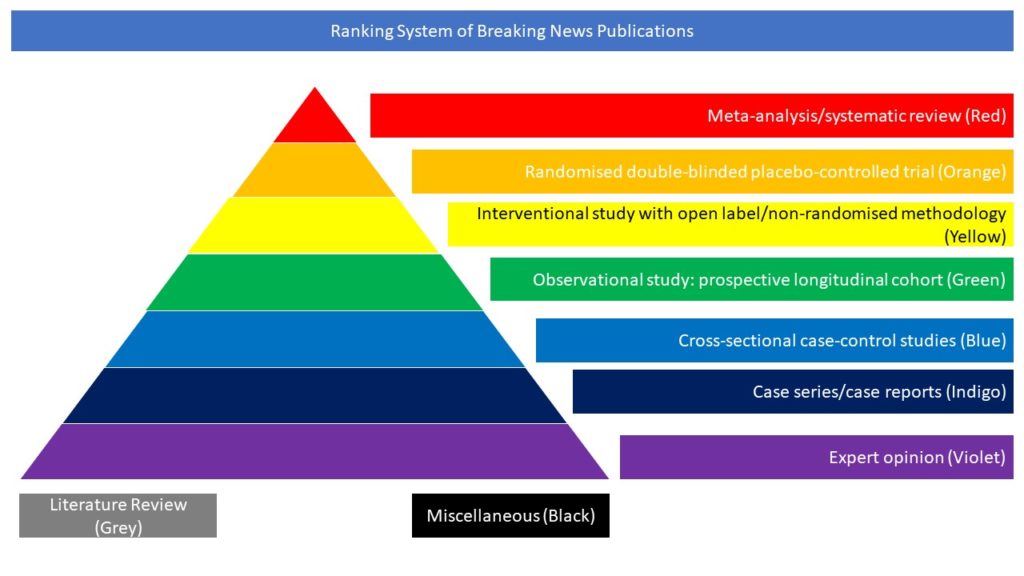
The description of every possible adverse effect or event related to vaccines is mandatory during the ongoing worldwide COVID-19 vaccination program. Although cases of cutaneous varicella zoster virus (VZV) reactivation after COVID-19 vaccination have been increasingly reported in literature and database sets, a description of VZV-induced neurological disease (VZV-ND) is still lacking. In this study, the authors retrospectively evaluated patients admitted to their clinic and diagnosed with VZV-ND during the COVID-19 vaccination campaign (January–April 2021) and in the same months in the previous two years. They identified three patients with VZV-ND after COVID-19 vaccination and 19 unvaccinated VZV-ND cases as controls. In the case–control analysis, the two groups showed no difference in clinical features, results of diagnostic investigations, and outcome. Thus, VZV reactivation with neurological involvement might be a possible event triggered by COVID-19 vaccination, but the benefit following COVID-19 vaccination overcomes significantly the potential risk associated with a VZV reactivation.
Abu-Rumeileh, S., Mayer, B., Still, V. et al. Varicella zoster virus-induced neurological disease after COVID-19 vaccination: a retrospective monocentric study. J Neurol (2021). https://doi.org/10.1007/s00415-021-10849-3.