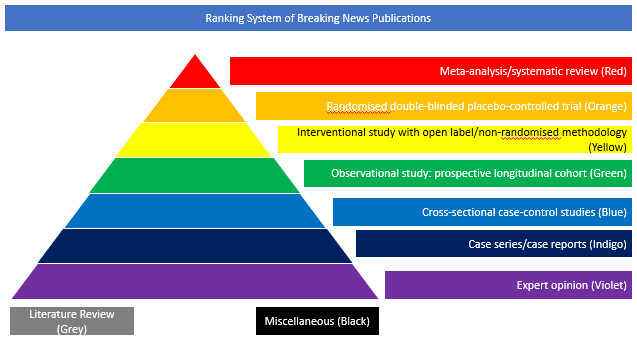
Cross-sectional case-control studies (Blue)
The objective of this study was to evaluate patient perspectives regarding the use of natalizumab and anti-CD20 therapies (rituximab and ocrelizumab) in the context of the COVID-19 pandemic. This cross-sectional study was conducted via voluntary survey filled in by patients with MS and related disorders receiving infusion-based treatment in one MS centre in Australia, exploring their concerns regarding their therapy and COVID-19, precautions undertaken in response to the pandemic, and factors impacting their decision-making. 170 patients completed the survey. Of patients taking natalizumab, the majority had either no or mild concern regarding their Disease Modifying Therapies (DMT) and COVID-19. Of patients treated with B-cell depleting therapies, again, the majority had no or mild concern, although a slightly higher proportion had a moderate level of concern. Asked to delineate their concerns, an increased risk of contracting COVID-19 was more commonly conveyed than MS-specific factors or poor outcomes pertaining to COVID-19 if contracted, by patients in both groups. Conversely, being invited to specifically consider the possibility of contracting COVID-19 or experience a relapse of MS, almost half of the cohort rated both of equal concern. More than half of the cohort were self-isolating more stringently than general government advice. Government-related resources followed by information provided by patient’s neurologists were reported as the most common means of obtaining information to guide decision making. The authors concluded that the attitudes expressed may underscore a willingness of patients to continue their DMT where benefits outweigh risks during future phases of the COVID-19 pandemic.
DOI: 10.1016/j.msard.2020.102516