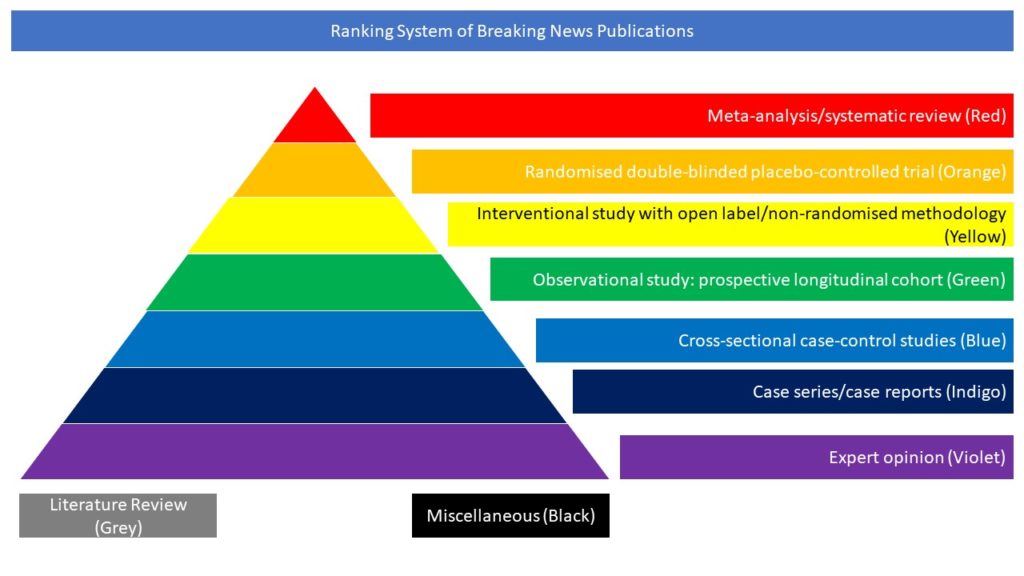
Randomised double-blinded placebo-controlled trial (Orange)
This randomized, double-blind, placebo-controlled phase 3 trial conducted in 99 amyotrophic lateral sclerosis (ALS) centres in 14 countries, tested the safety and efficacy of levosimendan, a calcium sensitizer that enhances myocyte contractility, in improving supine slow vital capacity after 12 weeks of treatment in 496 people with amyotrophic lateral sclerosis. Levosimendan did not improve respiratory function and overall functionality compared with placebo after 12 weeks of treatment, and was associated with some adverse events including increased heart rate, falls, headache and dysponoea.
Cudkowicz M, Genge A, Maragakis N, et al. Safety and efficacy of oral levosimendan in people with amyotrophic lateral sclerosis (the REFALS study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Neurol. 2021 Oct;20(10):821-831 doi: https://doi.org/10.1016/S1474-4422(21)00242-8.