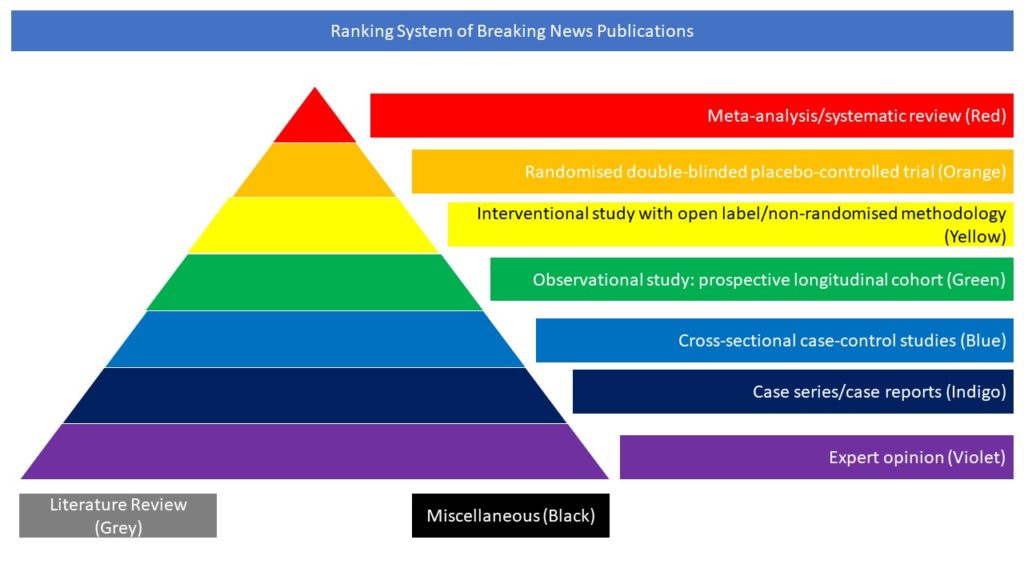
Case series/case reports (Indigo)
Whilst SARS-CoV-2 vaccines are very safe, in this brief communication the authors report four cases of the bifacial weakness with paraesthesias variant of Guillain-Barré syndrome (GBS) occurring within three weeks of vaccination with the Oxford-AstraZeneca SARS-CoV-2 vaccine. This rare neurological syndrome has previously been reported in association with SARS-CoV-2 infection itself. The patients were treated either with intravenous immunoglobulin, oral steroids, or no treatment. The authors suggest vigilance for cases of bifacial weakness with paraesthesias variant GBS following vaccination for SARS-CoV-2 and that post-vaccination surveillance programs ensure robust data capture of this outcome, to assess for causality.
Allen, C.M., Ramsamy, S., Tarr, A.W., Tighe, P.J., Irving, W.L., Tanasescu, R. and Evans, J.R. (2021), Guillain-Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. Accepted Author Manuscript. https://doi.org/10.1002/ana.26144