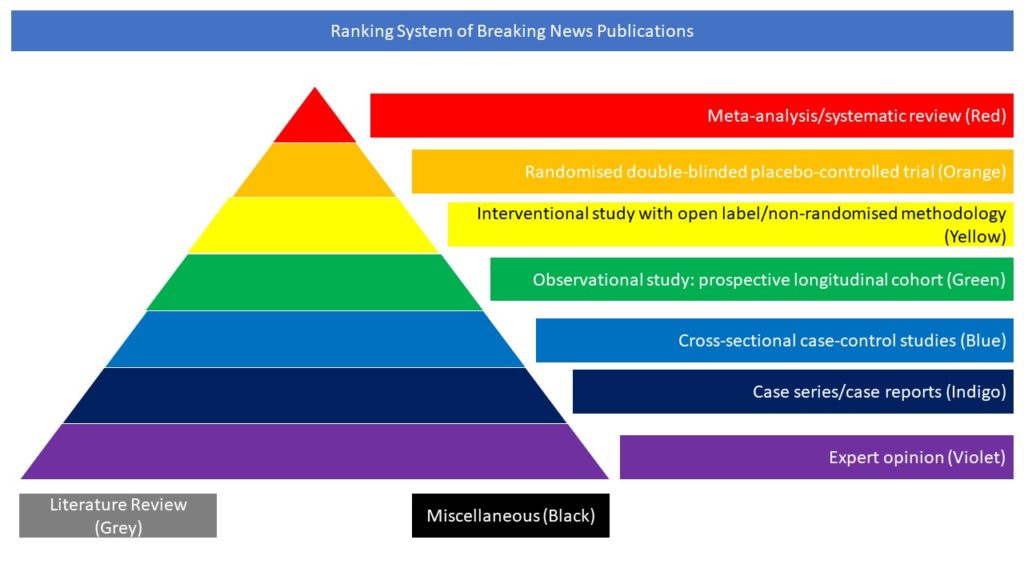
Observational study: prospective longitudinal cohort (Green)
As mass vaccination campaigns against COVID-19 commence worldwide, vaccine effectiveness needs to be assessed for a range of outcomes across diverse populations in a noncontrolled setting. In this study, data from Israel’s largest health care organization were used to evaluate the effectiveness of the BNT162b2 mRNA vaccine. All persons who were newly vaccinated during the period from December 20, 2020, to February 1, 2021, were matched to unvaccinated controls in a 1:1 ratio according to demographic and clinical characteristics. Study outcomes included documented infection with the SARS-CoV-2 , symptomatic COVID-19, COVID-19–related hospitalization, severe illness, and death. The authors estimated vaccine effectiveness for each outcome as one minus the risk ratio, using the Kaplan–Meier estimator. Each study group included 596,618 persons. Estimated vaccine effectiveness for the study outcomes at days 14 through 20 after the first dose and at 7 or more days after the second dose was as follows: for documented infection, 46% (95% confidence interval [CI], 40 to 51) and 92% (95% CI, 88 to 95); for symptomatic COVID-19, 57% (95% CI, 50 to 63) and 94% (95% CI, 87 to 98); for hospitalization, 74% (95% CI, 56 to 86) and 87% (95% CI, 55 to 100); and for severe disease, 62% (95% CI, 39 to 80) and 92% (95% CI, 75 to 100), respectively. Estimated effectiveness in preventing death from COVID-19 was 72% (95% CI, 19 to 100) for days 14 through 20 after the first dose. Estimated effectiveness in specific subpopulations assessed for documented infection and symptomatic COVID-19 was consistent across age groups, with potentially slightly lower effectiveness in persons with multiple coexisting conditions. The authors concluded that the BNT162b2 mRNA vaccine is effective for a wide range of COVID-19–related outcomes, a finding consistent with that of the randomized trial.
Dagan N, et al. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021 Feb 24. doi: 10.1056/NEJMoa2101765.