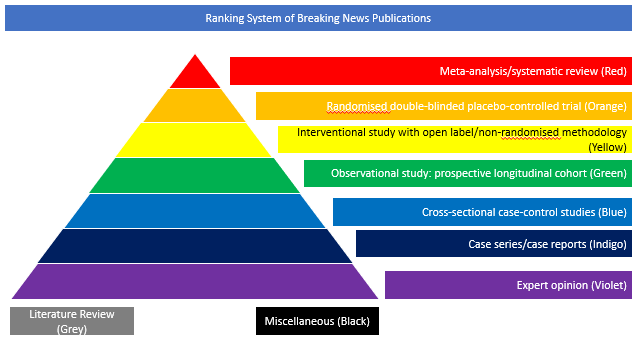
Cross-sectional case-control studies (Blue)
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been postulated to affect susceptibility to COVID-19. Observational studies so far have lacked rigorous ascertainment adjustment and international generalisability. In this international, open science, cohort analysis, the authors aimed to determine whether use of ACEIs or ARBs was associated with an increased susceptibility to COVID-19 in patients with hypertension. They used electronic health records from Spain (Information Systems for Research in Primary Care [SIDIAP]) and the USA (Columbia University Irving Medical Center data warehouse [CUIMC] and Department of Veterans Affairs Observational Medical Outcomes Partnership [VA-OMOP]) to identify patients aged 18 years or older with at least one prescription for ACEIs and ARBs (target cohort) or calcium channel blockers (CCBs) and thiazide or thiazide-like diuretics (THZs; comparator cohort) between Nov 1, 2019, and Jan 31, 2020. Users were defined separately as receiving either monotherapy with these four drug classes, or monotherapy or combination therapy (combination use) with other antihypertensive medications. Four outcomes were assessed: COVID-19 diagnosis; hospital admission with COVID-19; hospital admission with pneumonia; and hospital admission with pneumonia, acute respiratory distress syndrome, acute kidney injury, or sepsis. A large-scale propensity score methods were built derived through a data-driven approach and negative control experiments across ten pairwise comparisons, with results meta-analysed to generate 1280 study effects. For each study effect, the authors did negative control outcome experiments using a possible 123 controls identified through a data-rich algorithm. This process used a set of predefined baseline patient characteristics to provide the most accurate prediction of treatment and balance among patient cohorts across characteristics. Among 1 355 349 antihypertensive users (363 785 ACEI or ARB monotherapy users, 248 915 CCB or THZ monotherapy users, 711 799 ACEI or ARB combination users, and 473 076 CCB or THZ combination users) included in analyses, no association was observed between COVID-19 diagnosis and exposure to ACEI or ARB monotherapy versus CCB or THZ monotherapy (calibrated hazard ratio [HR] 0·98, 95% CI 0·84–1·14) or combination use exposure (1·01, 0·90–1·15). ACEIs alone similarly showed no relative risk difference when compared with CCB or THZ monotherapy (HR 0·91, 95% CI 0·68–1·21; with heterogeneity of >40%) or combination use (0·95, 0·83–1·07). Directly comparing ACEIs with ARBs demonstrated a moderately lower risk with ACEIs, which was significant with combination use (HR 0·88, 95% CI 0·79–0·99) and non-significant for monotherapy (0·85, 0·69–1·05). No significant difference between drug classes for risk of hospital admission with COVID-19, hospital admission with pneumonia, or hospital admission with pneumonia, acute respiratory distress syndrome, acute kidney injury, or sepsis across all comparisons were observed. The authors concluded that no clinically significant increased risk of COVID-19 diagnosis or hospital admission-related outcomes associated with ACEI or ARB use was observed, suggesting users should not discontinue or change their treatment to decrease their risk of COVID-19.
DOI: https://doi.org/10.1016/S2589-7500(20)30289-2