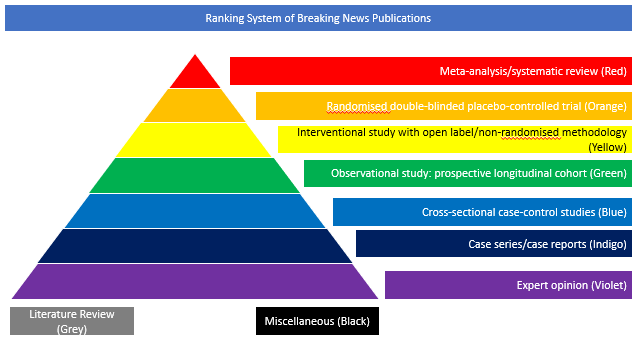
Randomised double-blinded placebo-controlled trial (Orange)
Recent data suggest that complications and death from COVID-19 may be related to high viral loads.
In this ongoing, double-blind, phase 1–3 trial involving non-hospitalised patients with COVID-19, the authors investigated two fully human, neutralising monoclonal antibodies against SARS-CoV-2 spike protein, given in a combined cocktail (REGN-COV2) hypothesised to reduce the risk of emergence of treatment-resistant mutant virus. Patients were randomly assigned (1:1:1) to receive placebo, 2.4 g of REGN-COV2, or 8.0 g of REGN-COV2 and were prospectively characterised at baseline for endogenous immune response against SARS-CoV-2 (serum antibody–positive or serum antibody–negative). Key endpoints included time-weighted average change from baseline in viral load from day 1 through day 7 and the percentage of patients with at least one COVID-19–related medically attended visit through day 29. Safety was assessed in all patients. Data from 275 patients were reported. The least-squares mean difference (combined REGN-COV2 dose groups vs. placebo group) in the time-weighted average change in viral load from day 1 through day 7 was −0.56 log10 copies per millilitre (95% confidence interval [CI], −1.02 to −0.11) among patients who were serum antibody–negative at baseline and −0.41 log10 copies per millilitre (95% CI, −0.71 to −0.10) in the overall trial population. In the overall trial population, 6% of the patients in the placebo group and 3% of the patients in the combined REGN-COV2 dose groups reported at least one medically attended visit; among patients who were serum antibody–negative at baseline, the corresponding percentages were 15% and 6% (difference, −9 percentage points; 95% CI, −29 to 11). The percentages of patients with hypersensitivity reactions, infusion-related reactions, and other adverse events were similar in the combined REGN-COV2 dose groups and the placebo group. In this interim analysis, the REGN-COV2 antibody cocktail reduced viral load, with a greater effect in patients whose immune response had not yet been initiated or who had a high viral load at baseline. Safety outcomes were similar in the combined REGN-COV2 dose groups and the placebo group.