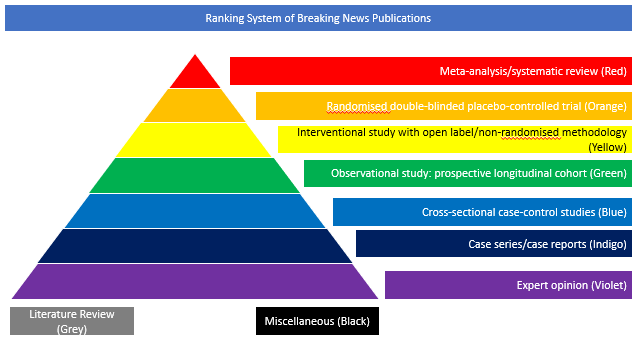
Randomised double-blinded placebo-controlled trial (Orange)
In this randomised, double-blind, placebo-controlled, single-dose trial conducted at 41 centres in the United States and involving outpatients with recently diagnosed mild or moderate COVID-19, the authors randomly assigned 452 patients to receive a single intravenous infusion of neutralising antibody LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo and evaluated quantitative virological endpoints and clinical outcomes. The primary outcome was the change from baseline in the viral load at day 11. The results of a preplanned interim analysis as of September 5, 2020, are reported in this paper. At the time of the interim analysis, the observed mean decrease from baseline in the log viral load for the entire population was −3.81, for an elimination of more than 99.97% of viral RNA. For patients who received the 2800-mg dose of LY-CoV555, the difference from placebo in the decrease from baseline was −0.53 (95% confidence interval [CI], −0.98 to −0.08; P=0.02), for a viral load that was lower by a factor of 3.4. Smaller differences from placebo in the change from baseline were observed among the patients who received the 700-mg dose (−0.20; 95% CI, −0.66 to 0.25; P=0.38) or the 7000-mg dose (0.09; 95% CI, −0.37 to 0.55; P=0.70). On days 2 to 6, the patients who received LY-CoV555 had a slightly lower severity of symptoms than those who received placebo. The percentage of patients who had a COVID-19–related hospitalisation or visit to an emergency department was 1.6% in the LY-CoV555 group and 6.3% in the placebo group. The authors concluded that in this interim analysis of a phase 2 trial, one of three doses of neutralising antibody LY-CoV555 appeared to accelerate the natural decline in viral load over time, whereas the other doses had not by day 11.