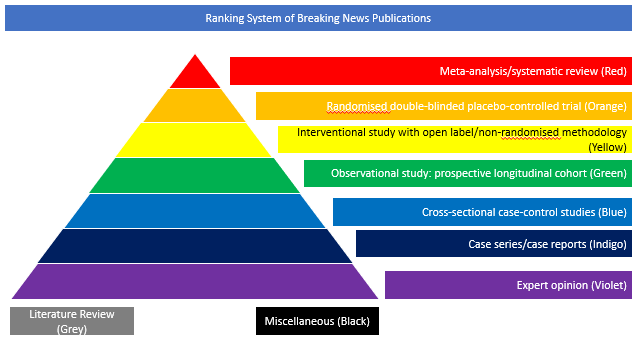
Randomised double-blinded placebo-controlled trial (Orange)
COVID-19 may lead to serious illness as a result of an excessive immune response. Fluvoxamine may prevent clinical deterioration by stimulating the σ-1 receptor, which regulates cytokine production. The objective of this double-blind, randomised, fully remote (contactless) clinical trial was to determine whether fluvoxamine, given during mild COVID-19 illness, prevents clinical deterioration and decreases the severity of disease. Participants were community-living, non-hospitalised adults with confirmed COVID-19 infection, with symptom onset within 7 days and oxygen saturation of 92% or greater. One hundred and fifty-two participants were enrolled. Participants were randomly assigned to receive 100 mg of fluvoxamine (n = 80) or placebo (n = 72) 3 times daily for 15 days. The primary outcome was clinical deterioration within 15 days of randomisation defined by meeting both criteria of (1) shortness of breath or hospitalisation for shortness of breath or pneumonia and (2) oxygen saturation less than 92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater. Of 152 patients who were randomised (mean [SD] age, 46 [13] years; 109 [72%] women), 115 (76%) completed the trial. Clinical deterioration occurred in 0 of 80 patients in the fluvoxamine group and in 6 of 72 patients in the placebo group (absolute difference, 8.7% [95% CI, 1.8%-16.4%] from survival analysis; log-rank P = .009). The fluvoxamine group had 1 serious adverse event and 11 other adverse events, whereas the placebo group had 6 serious adverse events and 12 other adverse events. The authors concluded that in this preliminary study of adult outpatients with symptomatic COVID-19, patients treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days. However, the study is limited by a small sample size and short follow-up duration, and determination of clinical efficacy would require larger randomised trials with more definitive outcome measures.
doi: 10.1001/jama.2020.22760