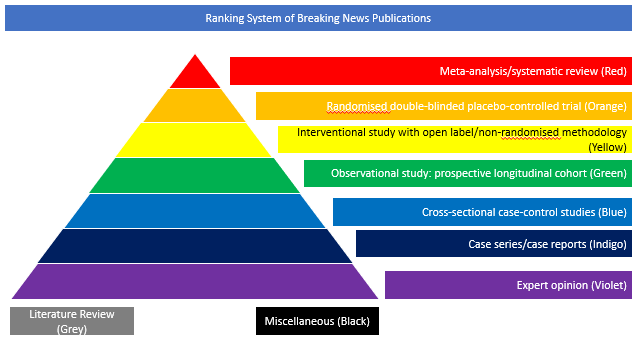
Interventional Study with Open Label/Non-Randomised Methodology (Yellow)
In this open-label, cluster-randomised trial involving asymptomatic contacts of patients with polymerase-chain-reaction (PCR)–confirmed COVID-19 in Catalonia, Spain, the authors randomly assigned clusters of contacts to the hydroxychloroquine group (which received the drug at a dose of 800 mg once, followed by 400 mg daily for 6 days) or to the usual-care group (which received no specific therapy). The primary outcome was PCR-confirmed, symptomatic COVID-19 within 14 days. The secondary outcome was SARS-CoV-2 infection, defined by symptoms compatible with COVID-19 or a positive PCR test regardless of symptoms. Adverse events were assessed for up to 28 days. The analysis included 2314 healthy contacts of 672 index case patients with COVID-19 who were identified between March 17 and April 28, 2020. A total of 1116 contacts were randomly assigned to receive hydroxychloroquine and 1198 to receive usual care. Results were similar in the hydroxychloroquine and usual-care groups with respect to the incidence of PCR-confirmed, symptomatic COVID-19 (5.7% and 6.2%, respectively; risk ratio, 0.86 [95% confidence interval, 0.52 to 1.42]). In addition, hydroxychloroquine was not associated with a lower incidence of SARS-CoV-2 transmission than usual care (18.7% and 17.8%, respectively). The incidence of adverse events was higher in the hydroxychloroquine group than in the usual-care group (56.1% vs. 5.9%), but no treatment-related serious adverse events were reported. The authors concluded that post-exposure therapy with hydroxychloroquine did not prevent SARS-CoV-2 infection or symptomatic COVID-19 in healthy persons exposed to a PCR-positive case patient.