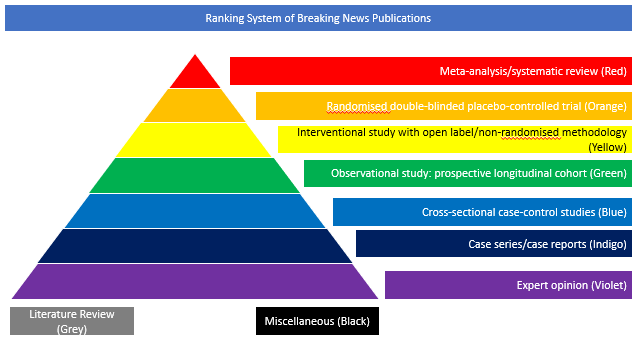
Interventional study with open label/non-randomised methodology (Yellow)
The objective of this open label, parallel arm, phase II, multicentre, randomised controlled trial, was to investigate the effectiveness of using convalescent plasma to treat moderate covid-19 in adults in India. 464 adults (≥18 years) admitted to hospital (screened 22 April to 14 July 2020) with confirmed moderate COVID-19 (partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2) ratio between 200 mm Hg and 300 mm Hg or a respiratory rate of more than 24/min with oxygen saturation 93% or less on room air) were enrolled. 235 were assigned to convalescent plasma with best standard of care (intervention arm) and 229 to best standard of care only (control arm) Participants in the intervention arm received two doses of 200 mL convalescent plasma, transfused 24 hours apart. The presence and levels of neutralising antibodies were not measured a priori; stored samples were assayed at the end of the study. The composite of progression to severe disease (PaO2/FiO2 <100 mm Hg) or all-cause mortality at 28 days post-enrollment represented the main outcome measures. Progression to severe disease or all-cause mortality at 28 days after enrollment occurred in 44 (19%) participants in the intervention arm and 41 (18%) in the control arm (risk difference 0.008 (95% confidence interval −0.062 to 0.078); risk ratio 1.04, 95% confidence interval 0.71 to 1.54). The authors concluded that convalescent plasma was not associated with a reduction in progression to severe COVID-19 or all-cause mortality. This trial has high generalisability and approximates convalescent plasma use in real life settings with limited laboratory capacity. A priori measurement of neutralising antibody titres in donors and participants might further clarify the role of convalescent plasma in the management of COVID-19.
DOI: https://doi.org/10.1136/bmj.m3939