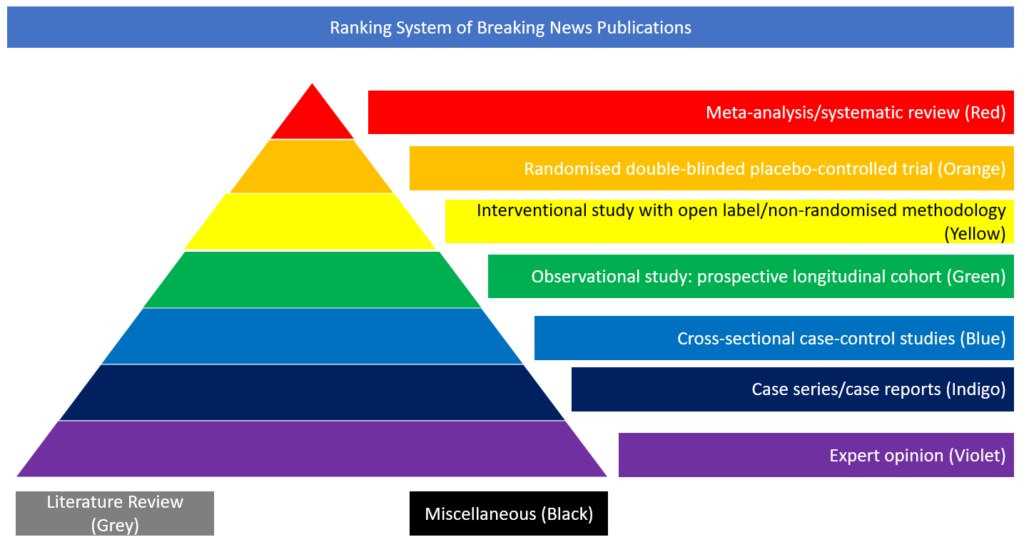
Interventional study with open label/non-randomised methodology
In this study recently published in the New England Journal of Medicine, the authors conducted a phase 1, dose-escalation, open-label trial including 45 healthy adults, 18 to 55 years of age, who received two vaccinations, 28 days apart, with candidate vaccine mRNA-1273 (which encodes the stabilised prefusion SARS-CoV-2 spike protein) in a dose of 25 μg, 100 μg, or 250 μg.
There were 15 participants in each dose group. After the first vaccination, antibody responses were higher with increasing doses (day 29 enzyme-linked immunosorbent assay anti–S-2P antibody geometric mean titer [GMT], 40,227 in the 25-μg group, 109,209 in the 100-μg group, and 213,526 in the 250-μg group). After the second vaccination, the titres increased in all dose groups (day 57 GMT, 299,751, 782,719, and 1,192,154, respectively). After the second vaccination, serum-neutralising activity was detected by two methods in all participants evaluated, with values generally similar to those in the upper half of the distribution of a panel of control convalescent serum specimens. Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site. Systemic adverse events were more common after the second vaccination, particularly with the highest dose, and three participants (21%) in the 250-μg dose group reported one or more severe adverse events. The authors concluded that the mRNA-1273 vaccine induced anti–SARS-CoV-2 immune responses in all participants, and no trial-limiting safety concerns were identified, supporting further development of this vaccine.
https://www.nejm.org/doi/full/10.1056/NEJMoa2022483