History Machado-Joseph disease (MJD) is an autosomal dominant (AD) spinocerebellar ataxia (SCA) initially described in descendants of immigrants from the Portuguese islands of the Azores. It was in 1972 that Kenneth K. Nakano and colleagues described the Machado family, with an AD, late onset, cerebellar ataxia with peripheral neuropathy. All family members were descendants from Guilherme Machado, who had migrated from São Miguel (Azores) to Massachusetts in the late nineteenth or early twentieth centuries. The Machado family has several American and Azorean generations. Later that year, Bryan T. Woods and Herbert H. Schaumburg described the Thomas family, descending from José Tomás (Joe Thomas after arriving in the USA) who had migrated from Flores (Azores) also to Massachusetts in the late nineteenth century. This family had an AD, early onset cerebellar ataxia with pyramidal syndrome, progressive external ophtalmoplegia and parkinsonism. In 1976 Roger N. Rosenberg and colleagues described the Joseph family, with an AD striatonigral degeneration. This family was descended from António Jacinto Bastiana (Antone Joseph after arriving in the USA) who ventured on a whaler trip from Flores to San Francisco Bay in 1844. At that time, the health system in the Azores was very poor and there were no neurologists in any of the nine islands of the archipelago. In 1975 the Portuguese health authorities asked Corino de Andrade to investigate this new disorder. Corino de Andrade, previously known for the original description of familial amyloid neuropathy type 1, invited Paula Coutinho to travel with him to the Azores and in a few months, they observed forty patients from fifteen families. In 1978 they reported the clinical features of all identified patients, acknowledged the continuum of clinical expression of this disorder and propose that the Machado, Thomas and Joseph families had the same genetic condition. The first pedigree not connected to the Azores was described in 1978 in mainland Portugal, and the first non-Portuguese family a year later, an American black family originating from North Carolina.
For years there had been a great controversy on the topic of one or several genetic disorders. It was in the “International Symposium on Autosomal Dominant Motor System Disorders in Persons of Portuguese Ancestry”, held in Lisbon in 1980, that the name Machado-Joseph disease was proposed by Paula Coutinho and Jorge Sequeiros.
Epidemiology. Population based epidemiological studies on SCA are scarce, with few data on the incidence or prevalence of each SCA subtype. However, there are numerous families in mainland Portugal and regions of high prevalence, namely the Tagus River Valley. MJD has also a high prevalence in Australian aboriginal communities (Groote Eylandt Island, Gulf of Carpentaria). Overall, the relative frequency is higher in China, Brazil, Portugal, Thailand, Germany, Singapore, Taiwan, France, Japan, The Netherlands, Venezuela, USA and Spain.
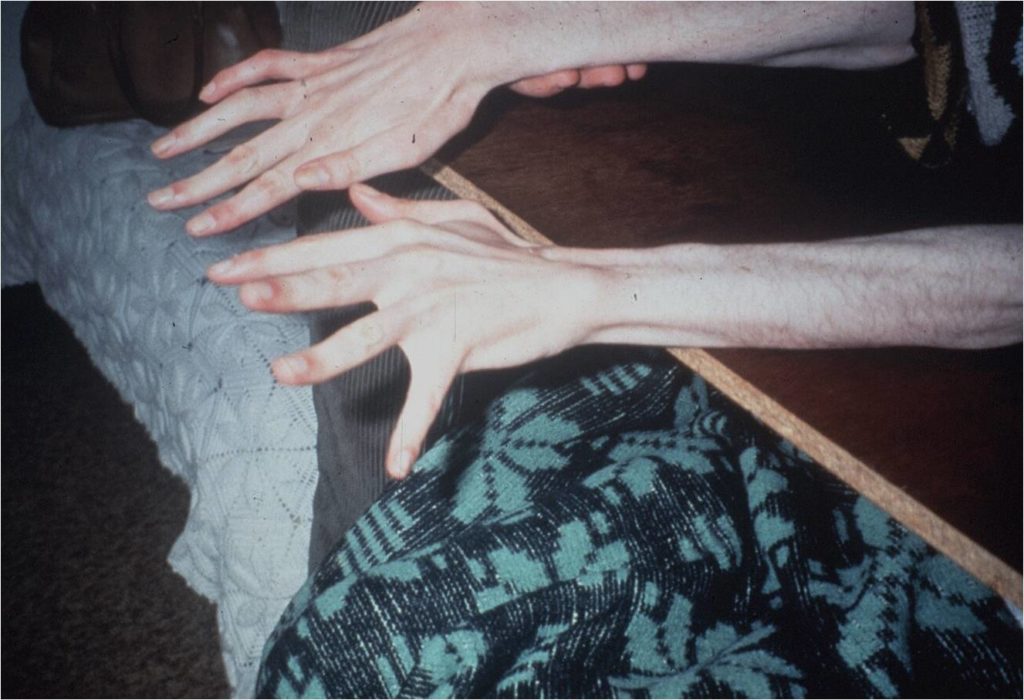
Clinical presentation. Onset is typically around 40 years-old, ranging from 7 until 70. A cerebellar syndrome with progressive external ophthalmoplegia is almost universal, independently of age of onset. In patients with young onset (<25 years old) there is a high prevalence of movement disorders (most frequently dystonia, but also Parkinsonism) and pyramidal signs, with a more aggressive disease course. In the intermediate phenotype pyramidal signs might also be present, with movement disorders and neuropathy being mild or absent. Late onset patients (³47 years old) present marked peripheral neuropathy, few or none pyramidal signs/ movement disorders, and have a slower disease progression.
Genetics. MJD diagnosis is established in the presence of an expanded allele in ATXN3 gene. Normal alleles range from 12 to 43CAG repeats, while fully penetrant expanded alleles have nearly always 60 CAG repeats or greater. As in other trinucleotide repeat disorders there is an inverse correlation between age of onset and number of repeats in the expanded allele. There is also a tendency for intergenerational repeat expansion, more evident in paternal transmission (not as striking as in other CAG expansion disorders). This accounts for some of the observed anticipation in subsequent generations.
Pathophysiology. Despite the mutation being identified in the early nineties, the pathogenic processes underlying MJD are still not completely understood. ATXN3 gene encodes ataxin 3, a widely expressed deubiquitinating enzyme that preferentially binds and cleaves longer chains of ubiquitin. In MJD, ataxin 3 contains an expanded polyglutamine tract that leads to conformational changes disabling its normal folding. Ataxin 3 becomes highly aggregation prone leading to its accumulation in the form of intranuclear inclusions, with aggregation associated toxicity dominating over folding and clearance.
Treatment. MJD is still a progressive disease for which there is currently no cure. Symptomatic treatments are available and physical therapy should be offered.
Future perspective. In rare diseases such as MJD, international networks are crucial. Data collection from large cohorts of carriers and patients will allow a deeper knowledge about MJD natural history as well as development of clinical, imaging and biological markers. Patients’ recruitment for clinical trials is easier among networks and hopefully a sufficiently powered clinical trial will be just around the corner.
Joana Damásio, José Barros
Hospital de Santo António, Centro Hospitalar do Porto (CHP) &
Instituto de Ciências Biomédicas Abel Salazar (ICBAS), Universidade do Porto
Porto, Portugal
joanadamasio@chporto.min-saude.pt
jb.neuro@chporto.min-saude.pt