The European Reference Network for Rare Neuromuscular Diseases (EURO-NMD) is a European Reference Network (ERN) of 61 Health Care Providers (HCPs) from 14 Member States with the ambition to cover all EU Member States. More than 100,000 NMD patients are seen annually by the ERN. This supports the European Directive 2011/24/EU on patient’s rights in cross-border healthcare. EURO-NMD meets a core aim of this directive to promote cooperation between health systems of Member States through implementation of an ERN.
Antonio Federico: What are the aims of your ERN?
Teresinha Evangelista: Our MISSION: Through collaborative cross-border work the EURO-NMD ERN will improve the lives of EU citizens by improving diagnosis, treatment and the delivery of high-quality, accessible and cost-effective healthcare to patients with rare Neuromuscular Diseases (NMD) requiring a particular concentration of resources or expertise.
The primary goals of EURO-NMD are:
- To improve quality and equity of healthcare for patients with rare neuromuscular diseases in all EU countries with appropriate outcomes for the specific NMD disease area;
- To make the best expertise and knowledge on NMD available to HCPs across Europe to ensure patients and healthcare professionals are well informed and up to date on best intervention and care options;
- To deliver better and faster diagnosis to NMD sufferers utilising state of the art techniques;
- To provide a web-based toolkit of combined resources based on expertise from across Europe that will support clinical services (diagnostics and care), research, training and education to stakeholders;
- To provide highly specialised services of high quality to help MS with low numbers of NMD patients or lacking technology or expertise (supporting Directive 2011/24/EU).
Antonio Federico: How is your ERN organised?
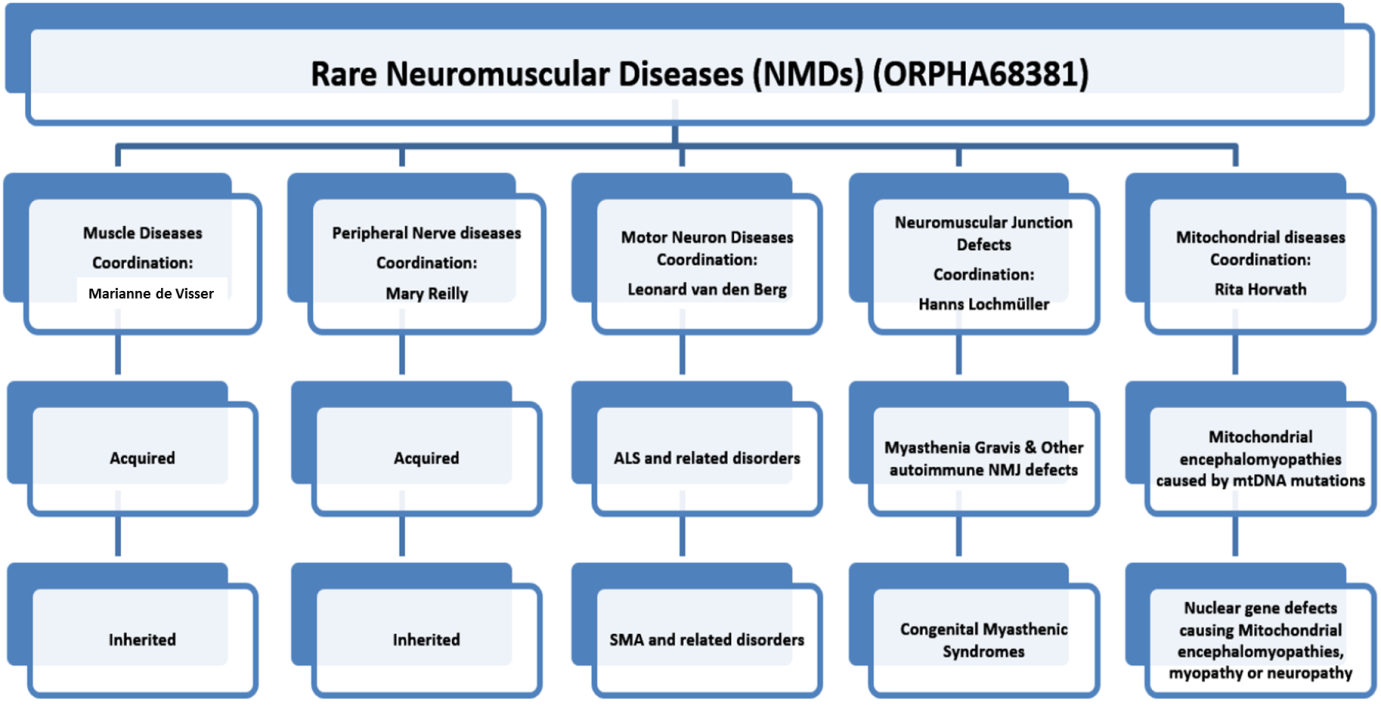
Teresinha Evangelista: EURO-NMD is organised around the following 5 thematic areas:
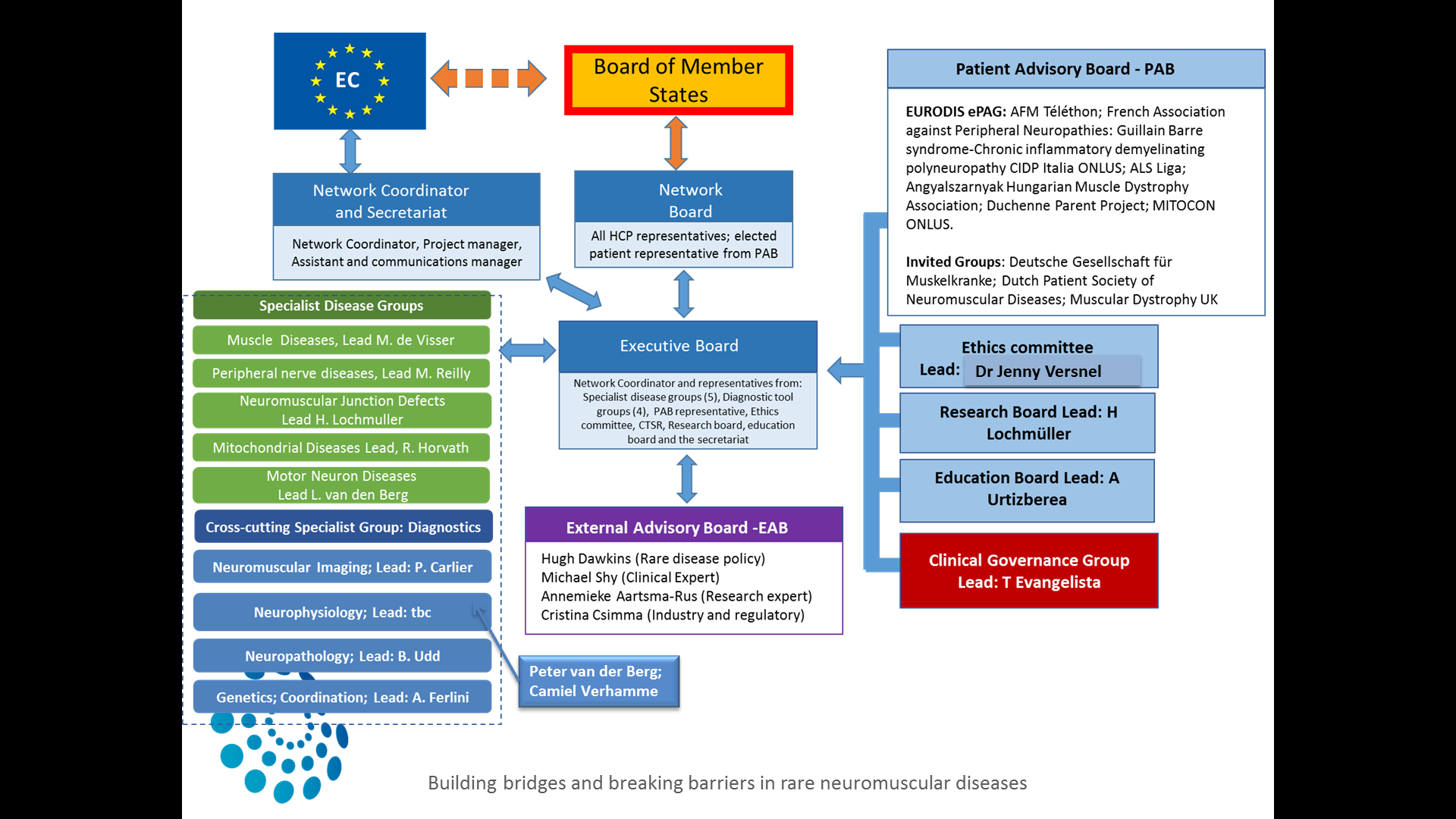
The BOARD of EURO-NMD is the primary decision-making body accountable for fostering collaboration; removing obstacles to the network successful functioning; and maintaining the focus of the network on the agreed scope, outcomes and benefits. The Board will be supported by the Executive Committee and by the Secretariat. The EXECUTIVE COMMITTEE (EC) is responsible for the overall policy and strategic direction, and oversees activities and progress.
The working groups of the network named Specialist Groups and Advisory Boards were organised as follows:
The five sub-thematic areas of the ERN (Muscle diseases, Neuropathies, Neuromuscular Junction Defects, Motor Neuron Diseases, and Mitochondrial diseases) will constitute the vertical Specialist Groups (SG). The SG for the sub-thematic areas will have working groups dedicated to care-guidelines, therapeutics, teaching, diagnostics and social care amongst others.
There are also cross cutting groups that cover the five disease areas; Diagnostic Tools Groups. These will cover the following four areas: genetics, neuromuscular pathology, imaging and neurophysiology and will address highly specialised interventions required for the diagnosis of NMD patients.shment of best practice guidelines, teaching and education in the respective areas.
The ERN work will also be supported by the following advisory boards: The Patient Advisory Board is responsible for identifying and acting on patients’ concerns and expectations. The Ethics Committee identifies and examines ethical and social aspects of clinical and research work. The Education Board identifies teaching needs and activities. The Research Board identifies and monitors research activities.
For clinical activities, there is a Steering group (SG) for clinical governance: The activity groups will appoint one or more members to take part in the screening of referrals to the network.
Antonio Federico: What activities do you plan for the next years?
Teresinha Evangelista:
GOAL: Deliver a high-profile, well-run, sustainable ERN over a 5-year period.
To ensure the effective coordination, governance and management of the EURO-NMD; To develop a sustainability plan by M48 with finance in place by M60 to ensure the sustainability of EURO-NMD.
GOAL: State of the art guidelines for standardized diagnosis, care and treatment for NMD patients to be available to and implemented in all member HCPs. Perform a GAP-analyse of what is available and prioritize the development, update or adoption of new or improved care guidelines for the majority of patients across the five target NMD disease areas and Diagnostic techniques.
GOAL: The best expertise and knowledge about NMDs and best intervention and care options are available to HCPs through electronic means to ensure equity of healthcare across Europe. The network aims to deliver a functioning communication system to support the implementation of clinical pathways. To build a knowledge base capturing data, epidemiological information and expertise in a way that may be shared easily for training, research or health and social care delivery to benefit patients
GOAL: The target healthcare professionals and patient groups are well educated about NMDs, and are up to date on the best diagnostic, intervention and care options. Map and tailor provision of NMD-specific courses and address gaps, define priority areas for teaching and training, develop online teaching materials, co-organize teaching courses and disease-specific master classes, and promote existing e-learning tools.
GOAL: Promote research activity within the network, ensure a harmonized baseline for research, and enable the standardization and reuse of network data and samples for research work.
Facilitate participation in clinical research, including patient registries, biobanks, natural history studies and clinical trials by an increasing number of NMD patients; To contribute to the development of better and cost-efficient diagnostic tools and to effective therapies for NMD through clinical and other research, e.g. burden of illness, health technology, preference and outcome studies; To provide information on planned and ongoing research activities to HCPs, clinicians and patients, to inform the development of guidelines and care pathways, and educational activities. To promote transnational data and sample sharing through ethically robust, high quality registries, biobanks and research data platforms.
Antonio Federico: Would you like to cooperate with EAN? If yes, how could this function?
Teresinha Evangelista: EURO-NMD would valorise the collaboration with the EAN. Extremely important areas of collaboration are the development of guidelines, education and training activities. EURO-NMD would like to interact with the rare diseases task force to help to increase the visibility of rare diseases across Europe. We would also look for collaboration with the Education Committee, the Guideline Production Group and the panels for Neuropathies, Muscle and Neuromuscular Junction Disorders and clinical Neurophysiology, as some of the work the ERN aims to do overlaps with the one done by these panels.
Interview by Professor Antonio Federico, Chair of the EAN Scientific Committee