by Maurizio A. Leone1, Magdalena Keindl2, Anthony H. Schapira3, Günther Deuschl4, Antonio Federico5
The EAN considers the production of neurological guidelines a major obligation, as this is a primary tool to improve clinical practice in neurology. The Scientific Committee (SC) of the EAN coordinates the production of guidelines through its 31 Subspecialty Scientific Panels (SSP) that include the excellence of European scientists in each subspecialty area.
The EAN has adopted the GRADE system (Grading of Recommendations, Assessment, Development and Evaluation) for guidelines production (Leone M.A., et al., European Journal of Neurology 2013; 20:410-9), and deems now necessary to revise the process of proposing, planning, developing and publishing guidelines in order to make it more clear and transparent.
Here we present practical suggestions to EAN members wishing to develop guidelines about treatment and diagnosis of neurological diseases within the frame of the EAN. We aim to make uniform, traceable and explicit the path from the decision to write an EAN guideline to its publication. We hope this will make easier a larger involvement of the EAN scientific community in producing guidelines.
Extensive information on the process of guideline proposal and production is reported in a paper in press (Leone M.A. et al. Practical recommendations for the process of proposing, planning, and writing a neurological management guideline by EAN task forces European Journal of Neurology, in press.).
Proposing, Planning and Writing an EAN Guideline
The SC together with the SSPs and the EAN board determine on the need for guidelines. The SC will proactively propose a list of guidelines. The EAN SSP or full members of the EAN can submit a proposal for guideline. EAN Guidelines will be produced by ad hoc Task Forces (TF) appointed by the SC.
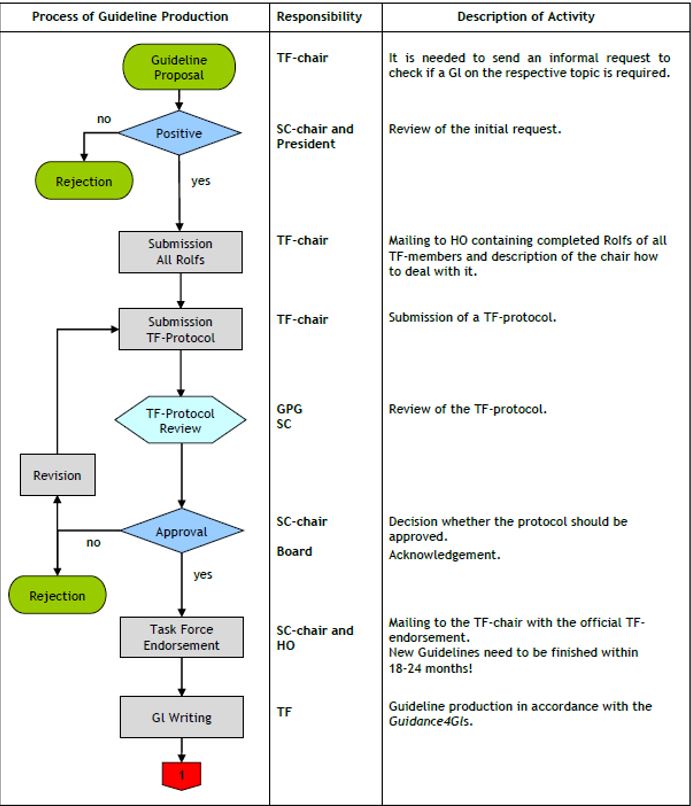
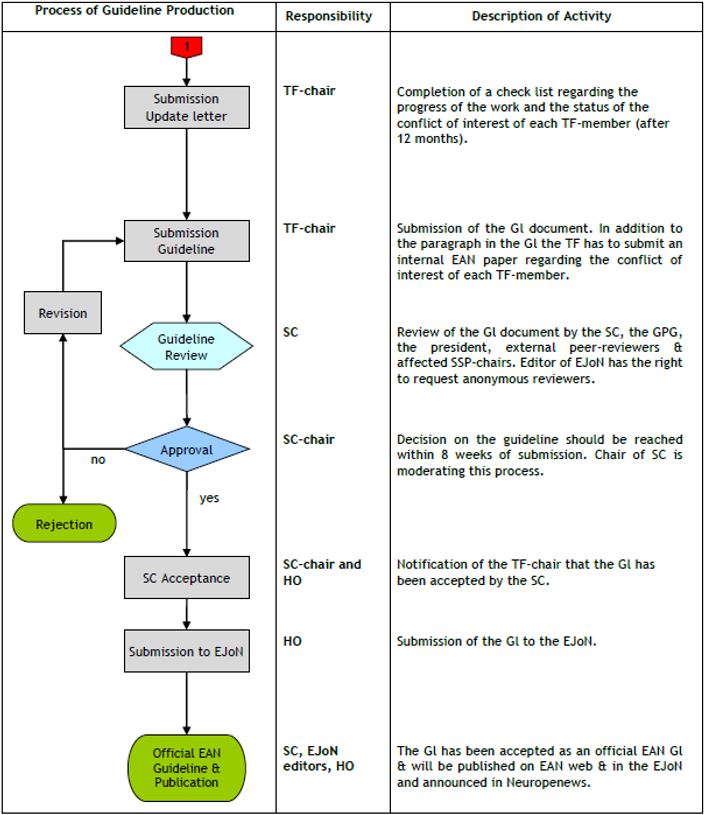
The flow-chart in Fig. 1 & 2 illustrate the whole process. Here we present a point-to-point explanation of the different steps.
1. Either upon request by the SC or due to the initiative of SSPs or individual members of the EAN a TF is created to propose an EAN guideline. The TF will consist of a chairperson and at least six but not usually more than 12 members. No more than two members should usually come from any one country. If feasible, the group should include a patient advocate (normally an officer from a European patient organization) if the TF deals with a clinically relevant topic, and other relevant specialists and health professionals.
2. The chair of the TF sends a guideline proposal to the chair of the SC and the EAN President including the title of the proposed guideline, the members of the TF, and a brief background illustrating the need of the guideline.
3. In case of acceptance, the TF chair collects the Register of Interests (ROI) of each TF member, including him/herself and send them to the Head Office of EAN. The ROI should describe any possible interest connected with the TF member that can or cannot be considered in conflict with the proposed guideline. The ROI form can be found here. Conflicts of interest (COI) are declared in the ROI form, including the recipient and amount of money. In case of interests in conflict with the proposed guideline, the TF chair must explain how the TF will deal with it. For example members who were involved in clinical studies with drugs cannot vote on questions related to these drugs. The SC will consider whether a COI is minor or major and will comment on the ways how to deal with it. EAN encourages that no more than half of the TF members do have any relevant COI related to the proposed guideline.
4. The TF chair submits a protocol for the guideline to the SC. The protocol should include the title, objectives, membership, a short explanation as to why the guideline is needed, already existing guidelines on the same or related topic, search strategy, method for reaching consensus, clinical questions and outcomes, and time frame for accomplishment. Clinical questions must be described in terms of Population, Intervention, Comparator and Outcome (PICO), according to GRADE [8]. A preliminary list and rating of outcome must be considered [8]. The search strategy should include the search string(s). The TF should identify in the protocol which tasks will be undertaken by each member, including those who will search the literature, prepare the evidence tables, grade the evidence and prepare the first draft of the guideline. The TF may apply to the EAN for financial support for the guideline production. In this case the TF must nominate a treasurer and submit an annual account to the EAN Board. The SC will encourage neurological specialty organisations to contribute to the guideline committee and to even co-author the guideline.
5. The guideline protocol is evaluated by the chair and the members of the SC and is approved, rejected, or a revision may be requested. The SC maintains a Guideline Production Group (GPG) which will comment on the methodological aspects of the guideline protocol. After final approval, and acknowledgment by the EAN board, the TF chair will be mailed the EAN endorsement. A list of the EAN guidelines under preparation will be placed on the EAN website. The TF has 18-24 months to complete the guideline.
6. The guideline production will follow the 2012 guidance paper [5]. A check list including all the steps of the guideline development according to GRADE will be sent to the TF chair at the beginning of the process. The GPG may assist the TF with methodological approach, including use of the GRADE system and way to reach consensus. It is also responsible for monitoring the entire flow of EAN guidelines.
7. The format of the guidelines will be that for the European Journal of Neurology, the official journal of the EAN, following a template with these sections:
- This should read: EAN Guideline on …… Report of EAN Task Force on ……… (title of Task Force, if different from the topic of the guideline);
- Authors should be listed with name of the chairperson first and the other authors in alphabetical order. The last name may be of a senior author. A possible alternative is “the EAN Task Force on management/diagnosis/other of condition” in which case a list of members follows.
- Structured abstract containing the main conclusions:
- Objectives;
- Background;
- Search strategy;
- Method for reaching consensus;
- Results;
- Recommendations;
- Statement of the time when the guidelines will likely need to be updated;
- Conflicts of interest;
- Role of each member of the TF
- References;
- Online material (e.g., summary of findings tables)
8. The length of the guideline report should be considered. While an ideal length is up to 6.000 words, it must be acknowledged that many guidelines deal with a number of questions and therefore need more space. In view of publication in the European Journal of Neurology additional files can be submitted for on-line only publication. Also supplementary material may be published on the EAN website.
9. In order to maintain a regular flow of guidelines and to ensure transparency, the GPG will request the TF chair to send an update on the progress of the work by filling the check list 12 months after the onset of the work. The TF chair will also be request to update on any new COI by him/her or the TF members at the completion of the guideline.
10. The TF will submit the completed guideline for approval to the chairperson of the SC, accompanied by a final update of the COIs, if any. The SC will have the proposed guideline reviewed by its members, the GPG, the President of the EAN, and the chairpersons of any Subspecialty Scientific Panels that might be affected by the guideline, although not involved in their preparation. External peer reviewing may be sought from content and methodological European and non-European experts. The Editor in Chief of the European Journal of Neurology (who is ex-officio member of the SC) has the right to ask for further anonymous review.
11. Within eight weeks from submission, the chairperson of the SC will notify the chairperson of the TF if the guideline has been accepted as official guideline of the EAN or not. If revision is needed, the TF will prepare a revised version and submit this to the review process again, highlighting the revisions and documenting the responses to each of the referees’ comments. Guidelines where the quality level does not qualify for EAN guidelines could be considered as an EAN consensus review.
12. Guidelines will be published in the European Journal of Neurology and the EAN website. The editor of the Journal may make minor editorial changes. Under exceptional circumstances, the EAN will allow simultaneous publication in the European Journal of Neurology and in the journal of the collaborating organisation of guidelines produced in agreement with other scientific societies, provided that advanced permission is sought prior to preparation of the Guideline. Under such circumstances the Guideline must be published simultaneously in the two journals.
13. Any new guideline will be announced in Neuropenews [9], the blog of the EAN. EAN members will be allowed to publish comments online.
14. National societies are encouraged to translate guidelines for dissemination in their own countries. Guidelines may be translated and published in local language journals. These translations have to refer to and to explain deviations from the original guideline, if any. Permission for translation must be sought from the EAN and Wiley, the publisher of the European Journal of Neurology. Translation must be checked and approved by the EAN Board.
15. The SC will regularly survey the validity of published guidelines and will ask for revision every 5 years or less if deemed necessary
1UOC Neurology, Department of Medical Sciences, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Italy.
2Department of Clinical Neurosciences and Preventive Medicine, Donau-Universität Krems, Austria.
3Department of Clinical Neurosciences, UCL Institute of Neurology, London, UK.
4Department of Neurology, University Hospital Schleswig-Holstein, Kiel Campus, Christian-Albrechts-University Kiel, Kiel, Germany.
5Department of Neurological, Neurosurgical and Behavioural Sciences, Medical School, University of Siena, Italy.