by Raja Sawaya and S. Hammoud
Abstract: We report another case of posterior reversible encephalopathy syndrome following Bevacizumab therapy for metastatic ovarian cancer. The prominent features of this report are the extension of the MRI changes to the anterior hemispheres and frontal lobes and the ease in controlling the seizures associated with this condition. We recommend renaming this syndrome as Benign Reversible Encephalopathy Syndrome (BRES) and avoiding unnecessary therapies for the syndrome or the seizures.
Introduction: Posterior reversible encephalopathy syndrome (PRES) is now a well described entity in neurology diagnosed by the clinical picture of altered mental status, headache, visual disturbances, hypertension and seizures, associated with the classical MRI findings of cortical and subcortical abnormalities in multiple brain areas predominantly in the posterior lobes.
This entity has been associated with eclampsia, hypertensive encephalopathy, neurotoxic drugs, immunosuppressants and electrolyte disturbances. (1)
We describe a case of PRES following bevacizumab therapy in a patient with metastatic ovarian carcinoma. We are presenting this case to highlight the benign nature of the seizure component of this syndrome and to suggest modifying its nomenclature to Benign Reversible Encephalopathy Syndrome (BRES) because most of the reported cases in the literature describe lesions in the frontal and temporal lobes and not only the posterior lobes and the prognosis for recovery from this condition is very good.
Case presentation: A 31-year-old female with metastatic high grade endometriod ovarian squamous cell carcinoma on multiple chemotherapy regimens presented with a focal tonic clonic seizure activity with secondary generalization 16 hours after receiving intravenous bevacizumab for the first time. The seizures were controlled with intravenous benzodiazepines, phenytoin and valproic acid. The patient was not known to have any previous neurological disorder or metastatic cerebral disease.
Neurological exam was consistent with a post ictal state following benzodiazepine treatment.
Cerebrospinal fluid analysis was normal with no malignant cells.
MRI of the brain showed abnormal high signal intensity on T2 and FLAIR sequences in the cortical and subcortical matter of the parietal and occipital lobes, posterior fossa, as well as the left pons, left cerebral peduncle and both frontal lobes. (Figure 1)
EEG revealed severe generalized cortical slowing with no ongoing epileptiform activity.
The patient recovered slowly over a fortnight and was discharged home seizure free to continue treatment of her ovarian cancer.
Discussion: PRES is an acute encephalopathy with diverse neurologic symptoms including headache, visual disturbance, altered mental status, hypertension and seizures.(1)
It is diagnosed by the clinical setting associated with typical MRI changes of hyperintense lesion on T2 and FLAIR sequences in multiple regions of the brain predominantly the posterior lobes and posterior fossa. (1,2) It has been associated with eclampsia, hypertensive crisis, chemotherapeutic agents, and immunosuppressant therapy and electrolyte disturbances. (1,2)
The pathophysiology of this syndrome is not clearly defined. One theory attributes hypertension and hyperperfusion to endothelial damage while a second theory suggests vasoconstriction and hypoperfusion as the trigger to generalized vasogenic edema of the cortex and subcortical white matter leading to the described MR image. (2-4)Bevacizumab is a recombinant humanized monoclonal IgG antibody which binds and inhibits vascular endothelial growth factors (VEGF) reducing angiogenesis and regressing tumor growth. (3-5)
PRES has been associated with Bevacizumab in multiple reported cases since 2006. (2-6) The common features in these reports and other papers describing this syndrome are the consistency of the symptoms complex, the reversibility of the clinical condition and MRI changes, the description of the MRI findings in the posterior structures and extension to the frontal lobes, the benign course of the seizure disorder and the self-remitting nature of this syndrome.
Considering the above, we suggest modifying the name of this syndrome to Benign Reversible Encephalopathy Syndrome (BRES) because the MRI features in most reports describe also lesions in the frontal lobes. Furthermore, we would like to emphasize that neither the syndrome itself nor the seizures entailed require treatment. The seizures resulting from the encephalopathy should be aborted by benzodiazepines therapy without the need for maintenance antiepileptic therapy. (1)
If the seizures recur or the patient develops persistent neurologic deficits we recommend abstaining from the diagnosis of BRES and searching for other causes for cerebral and cortical disease.
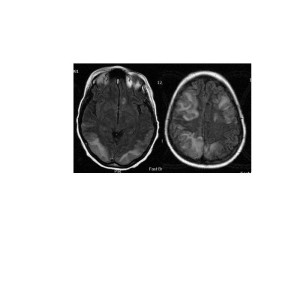
MRI brain, FLAIR sequence, axial images revealing hyperintense lesions posteriorly and frontally bilaterally.
1. Kastrup O, Gerwig M, Frings M, Diener HC. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012 Jul;259(7):1383-9.
2. Roth C, Ferbert A.The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Pract Neurol. 2011 Jun;11(3):136-44.
3. Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer.Arch Neurol. 2006 Oct;63(10):1475-8
4. Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006 Mar 2;354(9):980-2.
5. Tlemsani C, Mir O, Boudou-Rouquette P, Huillard O, Maley K, Ropert S, Coriat R, Goldwasser F. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target Oncol. 2011 Dec;6(4):253-8.
6. Seet RC, Rabinstein AA. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. QJM. 2012 Jan;105(1):69-75.
Raja Sawaya is Professor of Neurology at the American University Medical Centre in Beirut, Lebanon.
Comment by Wolfgang Grisold
This is an interesting article describing a case of PRES in a patient with ovarian carcinoma treated with bevacizumab. This case is exceptional as the frontal location of the presumed PRES changes is somewhat unusual. I would still not go as far as to re-name this syndrome, firstly because it is not always reversible and “benign”, and secondly the syndrome has a rather short history, and still not the whole neurological community is aware of this syndrome.
Concerning the proposed positive and benign recovery I would like to draw attention to the paper of Legriel S et al (1), who in a cohort of 70 patients found death in 16% and persisting functional impairment in 37%, and only 56% had a good outcome. In addition, although 88% of imaging findings were reversible, ischemic or haemorrhagic complications were noted in 14% and moreover a recurrence was noted in 5.7%. It should be also noted that a so called „severe PRES“ was described in this cohort requiring prolonged ventilation and as well suffered from a prolonged status epileptics.
It is important to acknowledge, that PRES occurs in multiple conditions, and in neuro-oncology also associations with other drugs, in particular platinum based drugs have been described. PRES has been observed also with cyclophosophamide, cytarabine, gemcitabine MTX, vincristine, bortezomib and chemotherapy combinations (2), possibly associated with intrathecal treatment (3) and rarely biological treatments as bevacizumab (4) and muromab (anti CD3 antibody).
Moreover also the association with immunosuppressants as several anticacineurin agents, high dose steroid treatment and possibly antiviral agents may be interesting in this context.
PRES can also occur as a presenting symptom of a tumour entity, which was demonstrated in a case report (5). This case was also examined autoptically and diffuse oedema in the absence of inflammation could be seen in the affected central white matters (for detailed autopsy and MR click the ECCO website) (Figure). The origin was not considered “paraneoplastic” as the patient had a severe hypercalcemia at presentation, and electrolyte changes have also been observed in association with PRES (6).
Concerning the clinical symptoms impairment of consciousness, seizures and headache seemed to be the most frequent symptoms, followed by nausea and sometimes focal neurological symptoms and signs. Definitely the occipital and parietal lobes were the most affected, often bilaterally (1). In neuro-oncological patients headache will be a leading syndrome and similarities between PRES and the SMART syndrome in previously radiated patients have been discussed (7).
The patient described here had a favourable course of the disease and it remains open whether the development of PRES was due to bevacizumab, the prior chemotherapy (which is not defined in the report) or a possible additional factor as hypertension, or a combination thereof. It is an interesting addition to the observed cases, but still the evidence of reports does not permit to call it benign.
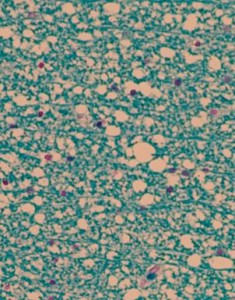
Autopsy from the affected white matter in a patient with PRES. Marked oedema in the white matter, giving it a “vacuolar “ appearance. No sign of inflammation (Klüver Myelin stain).
1 Legriel S SO, Azoulay E, Hantson P, Magalhaes E, et al. . Determinants of Recovery from Severe Posterior Reversible Encephalopathy Syndrome. PLoS ONE 7(9): e44534 doi:101371/journalpone0044534 2012.
2 Ramachandran A KR. Posterior Reversible Encephalopathy Syndrome (Pres) After Combination Chemotherapy for Lymphoma. Am J Ther 2012 Oct 16 [Epub ahead of print] 2012.
3 Aradillas E AR, Gasperino J. Methotrexate-induced posterior reversible encephalopathy syndrome. J Clin Pharm Ther 2011 Aug;36(4):529-36 doi: 101111/j1365-2710201001207x Epub 2010 Sep 30 2012.
4 Seet RC RA. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. QJM 2012 Jan;105(1):69-75 Epub 2011 Aug 24 2012.
5 Grisold W, Oberndorfer S. January’s Case Study: Coma, seizures, hypercalcemia and an unknown abdominal tumour. http://old.ecco-org.eu:// 06 Jan 2009
6 Kastrup MM, I. Wanke, H. C. Diener. Posterior reversible encephalopathy syndrome due to severe hypercalcemia. J Neurology ;249: 1563-1566.
7 Kerklaan JP, Lycklama á Nijeholt GJ, Wiggenraad RGJ, et al. SMART syndrome: a late reversible complication after radiation therapy for brain tumours. J Neurol. 2011 June; 258(6): 1098–1104.
Wolfgang Grisold is Professor of Neurology, WFN Trustee and Chair of the Department of Neurology at Kaiser-Franz-Joseph Hospital in Vienna, Austria